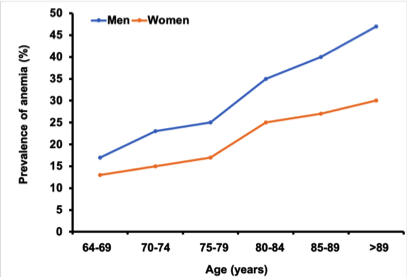
Roughly 17% of Canadian persons over the age of 65 is iron deficient or has iron deficiency anemia (IDA), and increases with advancing age. Many of the potential health consequences are reversible with restoration of healthy iron stores and hemoglobin/red blood cell (RBC) levels.
Iron is essential for many body functions throughout life. Symptoms of an iron deficiency may include reduced resistance to infections, impaired cognitive performance and behavior, fatigue, decreased exercise and work capacity, and chronically feeling cold.
Are you at risk of iron deficiency?
A wide range of causes and underlying diseases may lead to an iron deficiency or IDA, particularly as we age. It is not uncommon that more than one factor may contribute to the development of an iron deficiency or IDA. The most common causes of iron deficiency and IDA in Canada in persons of advancing age are inadequate intake of dietary iron, consumption of poorly available forms of iron, or diminished iron absorption. In addition, nutritional deficiencies in vitamin B12 and folate may contribute as well. The rate of vitamin B12 deficiency anemia in the elderly mirrors that of iron deficiency. The cause of iron deficiency and IDA are similar between men and women.
If you are at risk for iron deficiency or IDA, you should see your health care provider as soon as possible. Untreated iron deficiency or IDA may result in frailty and overall poor physical health; leading to an increased risk of falls and fracture, cognitive decline, and, ultimately, the loss of independent functioning. Your health care provider will examine you and measure your hemoglobin and serum ferritin. If iron deficiency or IDA is identified, oral elemental iron therapy will be initiated. The amount of elemental iron that you will be required to take will depend upon the severity of your iron deficiency or IDA.
Are all iron supplements the same?
No. Traditional elemental iron salt formulations (iron gluconate, iron sulfate, iron fumerate) required higher daily oral supplementation to maintain and correct an iron deficiency because of their poor bioavailability and absorption. In addition, these sources of elemental iron are associated with significant side effects such as constipation and gastrointestinal upset. A newer and safer source of elemental iron is iron (II) bisglycinate (a.k.a ferrous bisglycinate).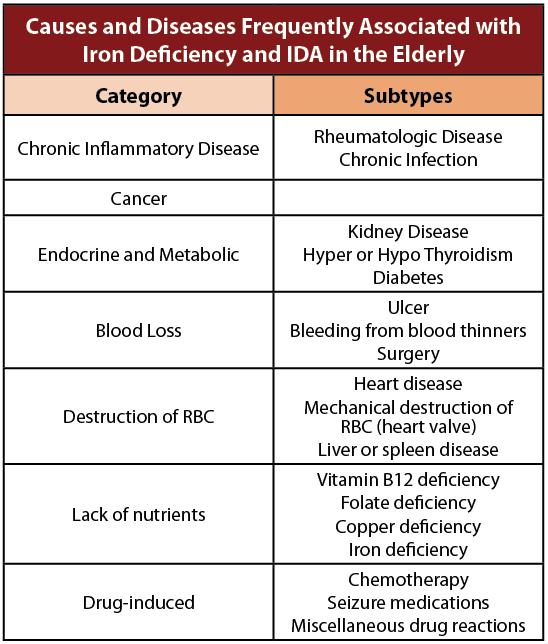
Iron (II) bisglycinate is made of only essential nutrients, iron chelated to two glycine molecules. It is not an iron salt. Iron (II) bisglycinate is absorbed 4.5x better than iron salts, resulting in a significant reduction in stomach upset, nausea, and constipation. Because of the improved absorption, a dose of 25 mg of iron (II) bisglycinate is equivalent to 50 mg of iron sulphate (an iron salt). Wouldn’t you prefer to take less?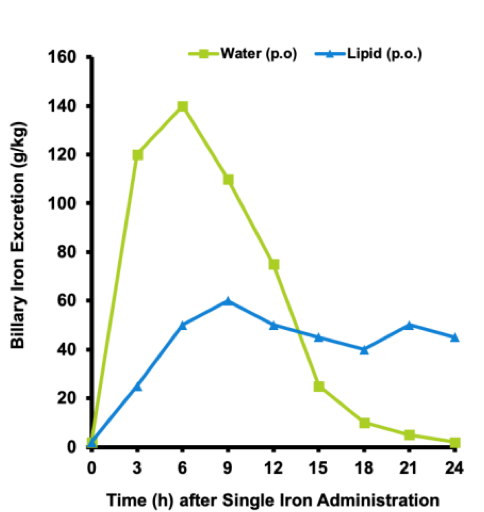
Are all formulations of oral supplemental iron the same?
No. Most oral iron supplements must be taken with a citrus drink to aid in stability and absorption. Many persons cannot tolerate the acidity of a citrus beverage. The acidity of citrus beverages are responsible for the irreversible erosion of dental enamel. Liquid iron formulations must be taken with a citrus beverage using a straw to avoid grey discolouration of your teeth. Tablet formulations may be problematic as we age because they may not always dissolve and may pass intact into the feces. Tablets may contain up to 90% excipients (binders, fillers, lubricants, and solubilizers) that may trigger unwanted side effects.
EasyIron® and EasyIron E.G ® are formulated with Iron (II) bisglycinate using Superior Nutrient Absorption (SNA). Excipient free!
SNA delivers iron (II) bisglycinate and other important nutrients suspended in healthy cold-pressed extra virgin olive oil in a vegetarian capsule– liquid in a capsule. Cold-pressed extra virgin olive oil has a natural source of vitamin E, making it the perfect natural preservative for iron (II) bisglycinate and the other important nutrients. SNA protects the stomach from the harshness of the iron and protects the vitamins from being degraded by the stomach acid. More importantly SNA ensures that all of the nutrients are easy to absorb by your body. SNA uses healthy olive oil to carry the nutrients across the intestinal cell membrane and into the cell unchanged. SNA ensures that all the nutrients are delivered to the liver and slowly released to the rest of our body throughout the day (blue line in the graph). Unlike tablet and liquid iron formulations that have peak concentrations in our body 5 to 6 hours after ingestion, (green line in the graph) and usually are taken 2 to 3 times per day, SNA supports the liver’s ability to slowly release iron (II) bisglycinate and important nutrients into our body over a 24 hour period. This ensures that iron (II) bisglycinate and its associated nutrients are available to your tissues throughout the day. You only have to take EasyIron® and EasyIron E.G ® once a day.



